Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery
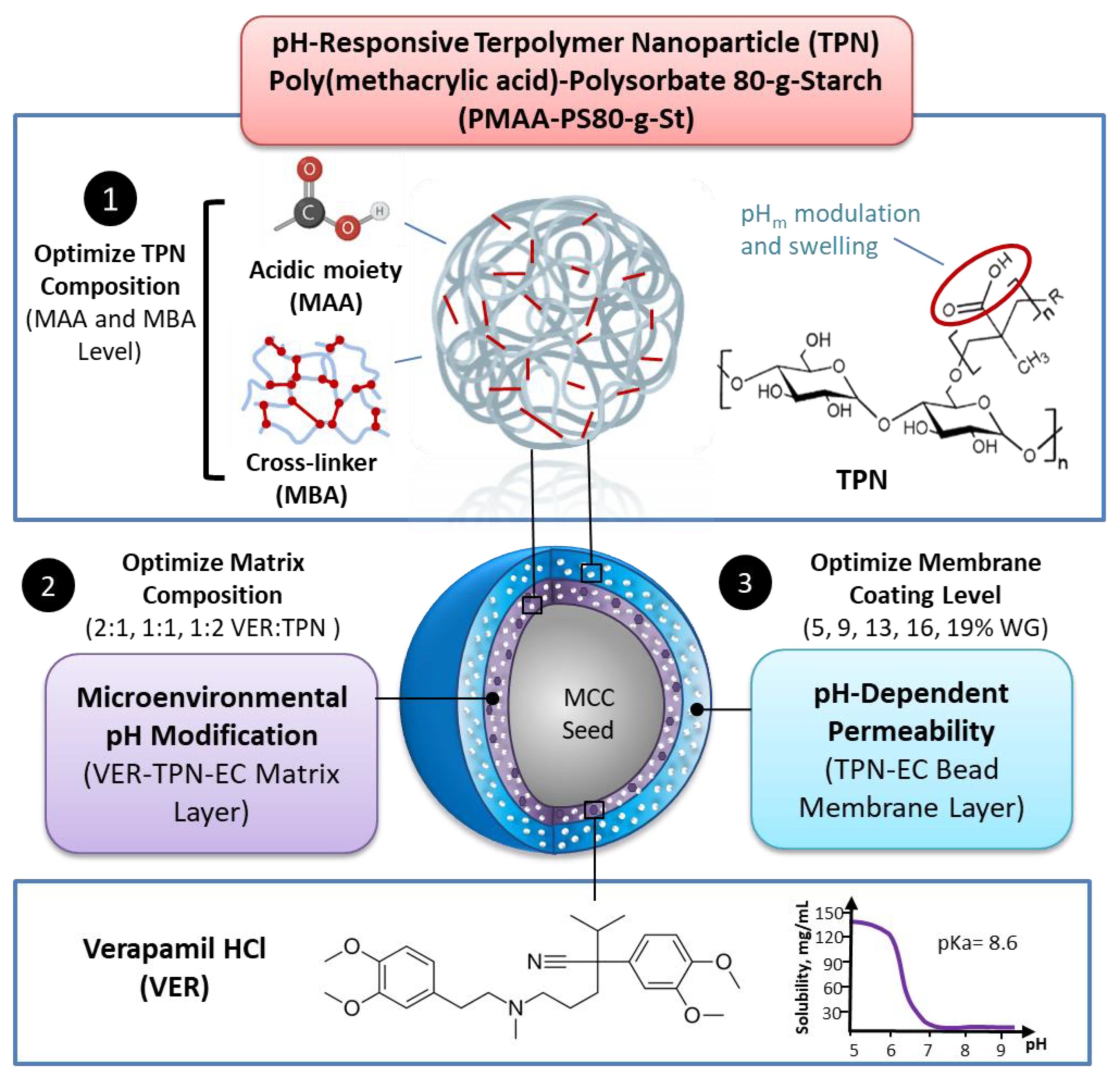
Bioavailability of weakly basic drugs may be disrupted by dramatic pH changes or unexpected pH alterations in the gastrointestinal tract. Conventional organic acids or enteric coating polymers cannot address this problem adequately because they leach out or dissolve prematurely, especially during controlled release applications. Thus, a non-leachable, multifunctional terpolymer nanoparticle (TPN) made of cross-linked poly(methacrylic acid) (PMAA)-polysorbate 80-grafted-starch (PMAA-PS 80-g-St) was proposed to provide pH transition-independent release of a weakly basic drug, verapamil HCl (VER), by a rationally designed bilayer-coated controlled release bead formulation.
The pH-responsive PMAA and cross-linker content in the TPN was first optimized to achieve the largest possible increase in medium uptake alongside the smallest decrease in drug release rate at pH 6.8, relative to pH 1.2. Such TPNs maintained an acidic microenvironmental pH (pHm) when loaded in ethylcellulose (EC) films, as measured using pH-indicating dyes. Further studies of formulations revealed that with the 1:2 VER:TPN ratio and 19% coating weight gain, bilayer-coated beads maintained a constant release rate over the pH transition and exhibited extended release up to 18 h. These results demonstrated that the multifunctional TPN as a pHm modifier and pH-dependent pore former could overcome the severe pH-dependent solubility of weakly basic drugs.
Download the full article as PDF here: Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery
or read it here
2.1. Materials
Chang, H.H.R.; Chen, K.; Lugtu-Pe, J.A.; AL-Mousawi, N.; Zhang, X.; Bar-Shalom, D.; Kane, A.; Wu, X.Y. Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery. Pharmaceutics 2023, 15, 547.
https://doi.org/10.3390/pharmaceutics15020547

