Amorphous nasal powder advanced performance: in vitro/ex vivo studies and correlation with in vivo pharmacokinetics
Abstract
Purpose
Amorphous solid dispersions (ASD) for nasal delivery offer the opportunity to increase drug release performance, while using polymers with mucoadhesive properties. The aim of the present study was to apply this solubility enhancement technique to a poorly soluble drug for nasal delivery, while comparing two particle engineering strategies, namely spray dried microparticles and chimeral agglomerates, with the corresponding physical blends with crystalline drug.
Methods
Formulations of piroxicam were manufactured using varied polymer and particle engineering strategies and evaluated through in vitro drug release and ex vivo permeation studies, as well as nasal deposition and in vivo pharmacokinetic studies.
Results
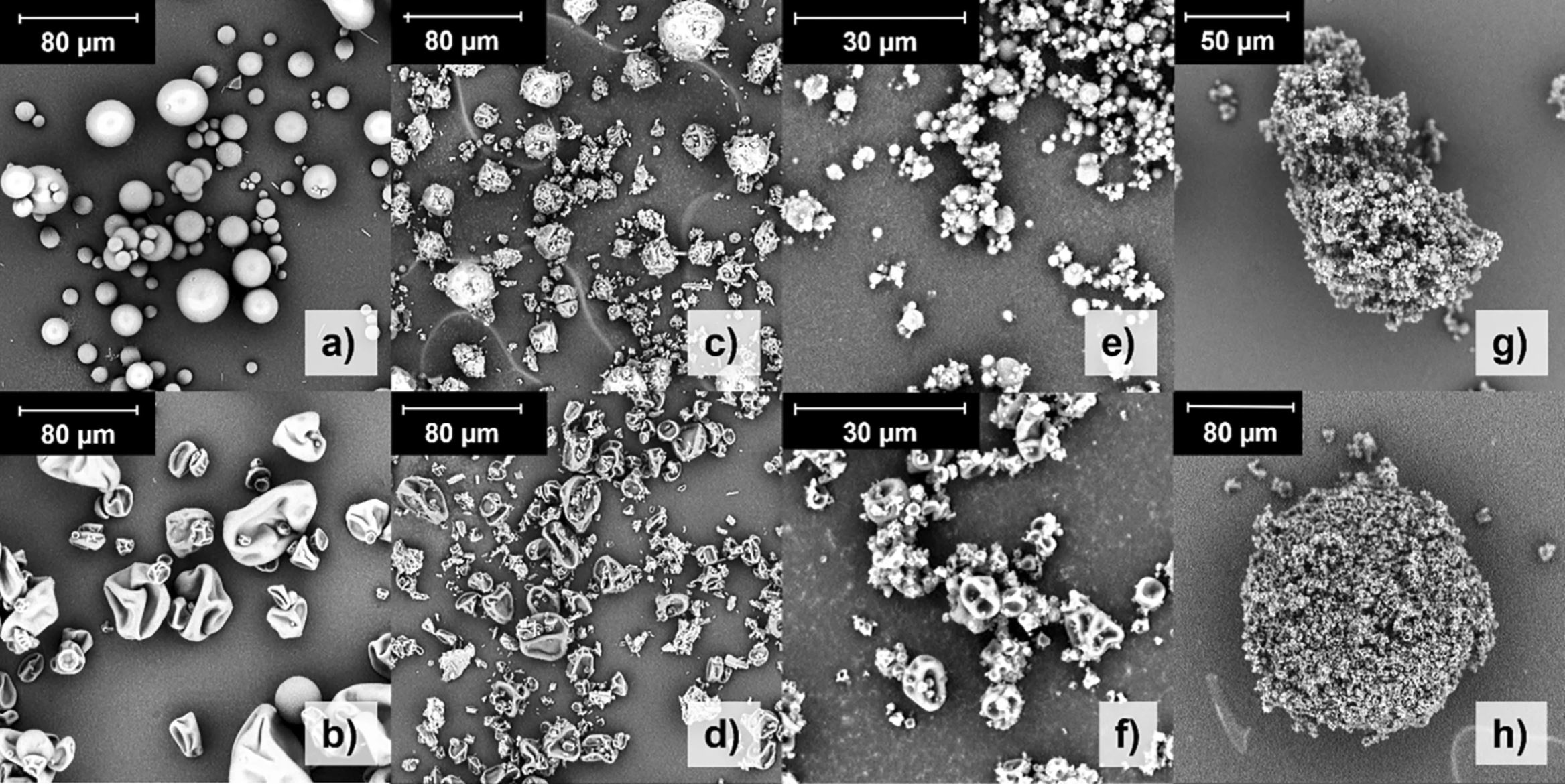
ASD with hydroxypropyl methylcellulose (HPMC) showed enhanced drug release and permeation, compared to polyvinylpyrrolidone/vinyl acetate formulations and blends. Nasal deposition of HPMC chimeral agglomerates suggested off-target deposition. In vivo pharmacokinetic studies revealed that spray-dried HPMC-containing microparticles exhibited the highest maximum plasma concentration (Cmax) and the lowest time to attain it (tmax). In vitro release rate and in vivo absorption rate were correlated as well as tmax and in vitro performance. When excluding the formulation with least nasal targeted deposition, in vitro release and ex vivo permeation performance were also correlated with Cmax and area under the drug concentration-time curve (AUC) from 0 to 1 h, with R2 > 0.89.
Conclusion
ASD for nasal delivery provide fast drug absorption, which depends on the supersaturation ability of the polymer employed. In vitro-in vivo correlations suggested that in vitro release and ex vivo permeation studies are predictive tools regarding nasal absorption.
Download the full article as PDF here Amorphous nasal powder advanced performance: in vitro/ex vivo studies and correlation with in vivo pharmacokinetics
or read it here
Materials
PXC was purchased from abcr GmbH (Karlsruhe, Germany). Hydroxypropyl methylcellulose (HPMC) (grade E3, substitution type 2910) was provided by Dow Europe (Horgen, Switzerland) and polyvinylpyrrolidone/vinyl acetate (PVP/VA) by Ashland Specialties (Beveren, Belgium). Water from Milli-Q® Water Purification System (Millipore®, MA, USA) was used and other solvents as acetonitrile, methanol (Merck KGaA, Darmstadt, Germany) and dimethyl sulfoxide (DMSO) (Fisher Scientific, Loughborough, UK) were from analytical or high-performance liquid chromatography (HPLC) grade. Unless otherwise specified, all remaining chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
See the webinar:
“Fast Track development of Biphasic nano-dexamethasone Pellets using galenIQ™”, 12 October 2023:
Get more information & register here for free:


