Nanostructured Lipid Carriers for Oral Treatment of Leishmaniasis: Design and Preclinical Evaluation
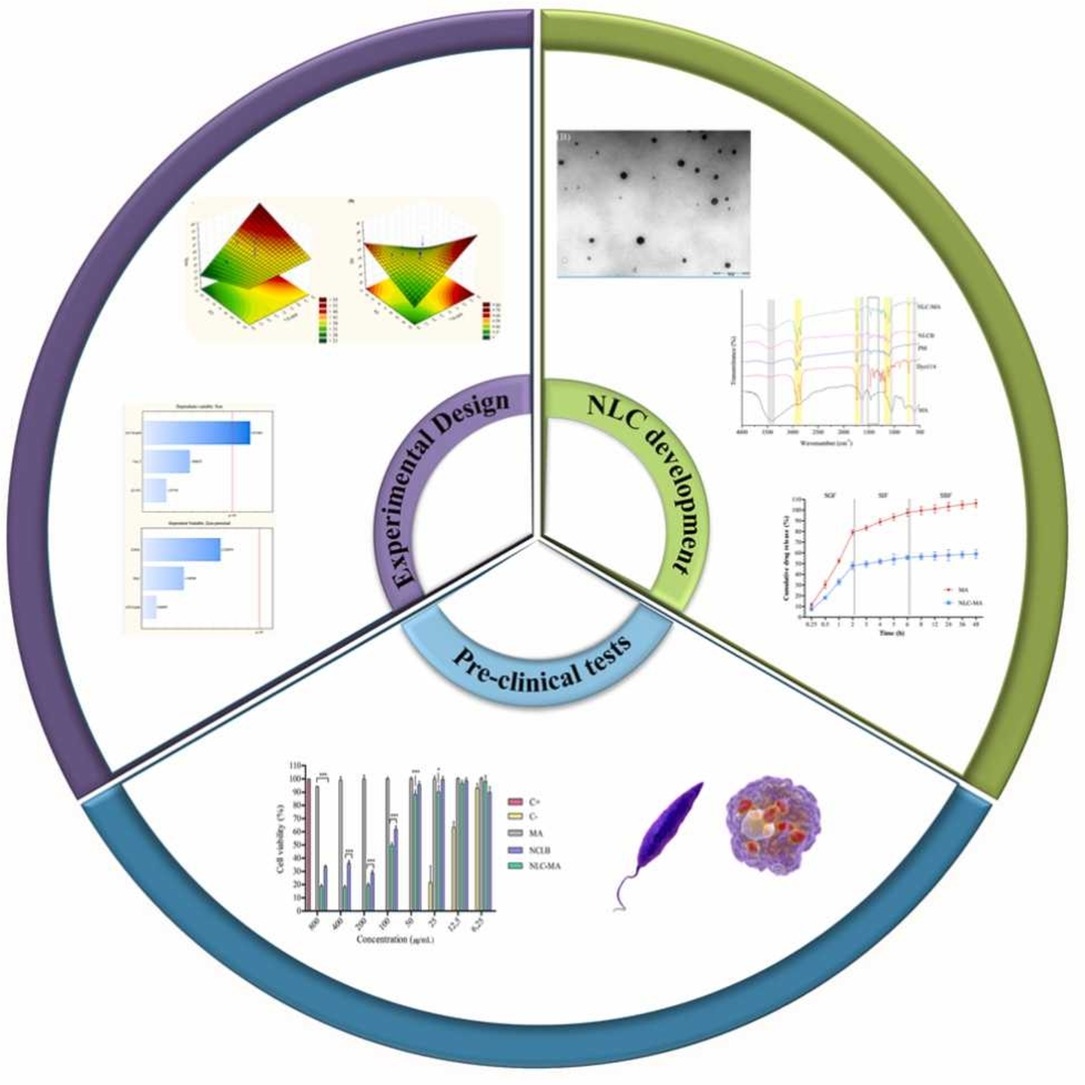
Pentavalent antimonials are the primary treatment for leishmaniasis due to their proven efficacy and cost-effectiveness. However, they present poor oral absorption, parenteral administration, and serious adverse effects due to prolonged use. Nanostructured Lipid Carriers (NLC) offer a promising solution, providing prolonged release, enhanced intestinal permeability, and oral bioavailability. This study aimed to address these issues by developing Nanostructured Lipid Carrier loaded with meglumine antimoniate (NLC-MA) for oral leishmaniasis treatment. We used design of experiments (DoE) for optimization.
Highlights
- The design of experiments provided better lipid formulation for the nanoparticle.
- Nanostructured Lipid Carrier containing meglumine antimoniate was developed.
- Simulated gastric stability of the nanocarrier was observed.
- The nanocarrier promoted in vitro release kinetics longer than 48 h.
- In vitro leishmanicidal activity was better in the L. infantum specie.
The chosen NLC-MA was characterized assessing size, polydispersity index (PdI), zeta potential (ZP), entrapment efficiency (EE), pH, and by analytical techniques such as DSC, FTIR, XRD, and TEM. Stability under simulated gastrointestinal conditions, in vitro release kinetics, cytotoxicity in RAW 264.7 macrophages, and leishmanicidal activity were assessed. NLC-MA presented 41.13 nm, PdI 0.227, ZP -27.9 mV, EE of 62.43%, and pH 5.23. Characterization techniques confirmed drug incorporation and nanoparticles with reduced crystallinity. TEM images showed spherical morphology. NLC remained stable in gastrointestinal pH and under refrigeration storage, while lyophilized formulations retained their initial properties.
MA release from NLC followed the Peppas-Sahlin model, an anomalous transport mechanism. Importantly, MA showed no cytotoxicity in macrophages, but the NLCs exhibited cytotoxicity beyond 50 µg/mL. NLC-MA displayed improved efficacy against L. infantum. Overall, these results highlight the potential of utilizing a lipid nanocarrier incorporating meglumine antimoniate as an innovative drug delivery platform for oral treatment against leishmaniasis.
Read more here
Materials
Dynasan® 114 (trimyristin) and Miglyol® 812 (caprylic/capric triglyceride) were generously provided by IOI Chemical (Hamburg, Germany); Compritol® ATO 888 (glyceryl behenate) was obtained from MCassab (São Paulo, Brazil); Alkest CSO 300 (ethoxylated castor oil OE30) was acquired from Oxiteno (São Paulo, Brazil); Span™ 80 (sorbitan monooleate) was purchased from Sigma (St Louis, USA); meglumine antimoniate (MA) was purchased from Acros Organics (Geel, Belgium, batch A0374354).
Myla Lôbo de Souza, Victor de Albuquerque Wanderley Sales, Samilly Gabrielly dos Santos Sales, Policarpo Ademar Sales Júnior, Valéria Rêgo Alves Pereira, Elvis Joacir de França, Larissa de Araújo Rolim, Pedro José Rolim Neto,
NANOSTRUCTURED LIPID CARRIERS FOR ORAL TREATMENT OF LEISHMANIASIS: DESIGN AND PRECLINICAL EVALUATION, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 134140, ISSN 0927-7757, https://doi.org/10.1016/j.colsurfa.2024.134140.
Read more interessting articles on Leishmaniasis here:
- Efficacy of topical risedronate and risedronate – Eudragit E complex in a model of cutaneous leishmaniasis induced by Leishmania (Leishmania) amazonensis
- Current status of nanoscale drug delivery and the future of nano-vaccine development for leishmaniasis
- Plant Bioactive Ingredients in Delivery Systems and Nanocarriers for the Treatment of Leishmaniasis