Evaluation of a Three-Fluid Nozzle Spraying Process for Facilitating Spray Drying of Hydrophilic Polymers for the Creation of Amorphous Solid Dispersions
Abstract
Amorphous solid dispersions (ASDs) enable formulations to improve the solubility of poorly soluble active pharmaceutical ingredients (APIs). The amorphous state is reached through the disruption of the crystalline lattice of an API resulting in an increased apparent solubility with faster disintegration. Nevertheless, this form is characterized by a high-energy state which is prone to re-crystallization. To ensure a stable ASD, excipients, e.g., polymers that form a matrix in which an API is dispersed, are used. The applicable polymer range is usually linked to their solubility in the respective solvent, therefore limiting the use of hydrophilic polymers. In this work, we show the applicability of the hydrophilic polymer, polyvinyl alcohol (PVA), in spray-dried solid dispersions. Using a three-fluid nozzle approach, this polymer can be used to generate ASDs with a targeted dissolution profile that is characterized by a prominent spring and desired parachute effect showing both supersaturation and crystallization inhibition. For this purpose, the polymer was tested in formulations containing the weakly basic drug, ketoconazole, and the acidic drug, indomethacin, both classified as Biopharmaceutics Classification System (BSC) class II drugs, as well as the weakly basic drug ritonavir classified as BCS IV. Furthermore, ritonavir was used to show the enhanced drug-loading capacity of PVA derived from the advantageous viscosity profile that makes the polymer an interesting candidate for spray drying applications.
Introduction
In recent years, the creation of amorphous solid dispersions (ASD) has emerged as a promising strategy to enhance both the solubility and bioavailability of low water-soluble drug substances. The creation of such formulations usually involves the dispersion of a drug substance within a polymer matrix at a molecular level resulting in a homogenous amorphous mixture [1].
As ASDs are inherently unstable and tend to crystallize over time, one of the key challenges is maintaining the amorphous state during storage and handling. To overcome this limitation, various techniques have been explored to stabilize ASDs after production.
Mainly three preparation methods are used to create ASDs: melting methods, solvent evaporation, and mechanical activation methods. Different preparation methods also result in varying thermal histories and the amount of mechanical stress introduced into the material [2].
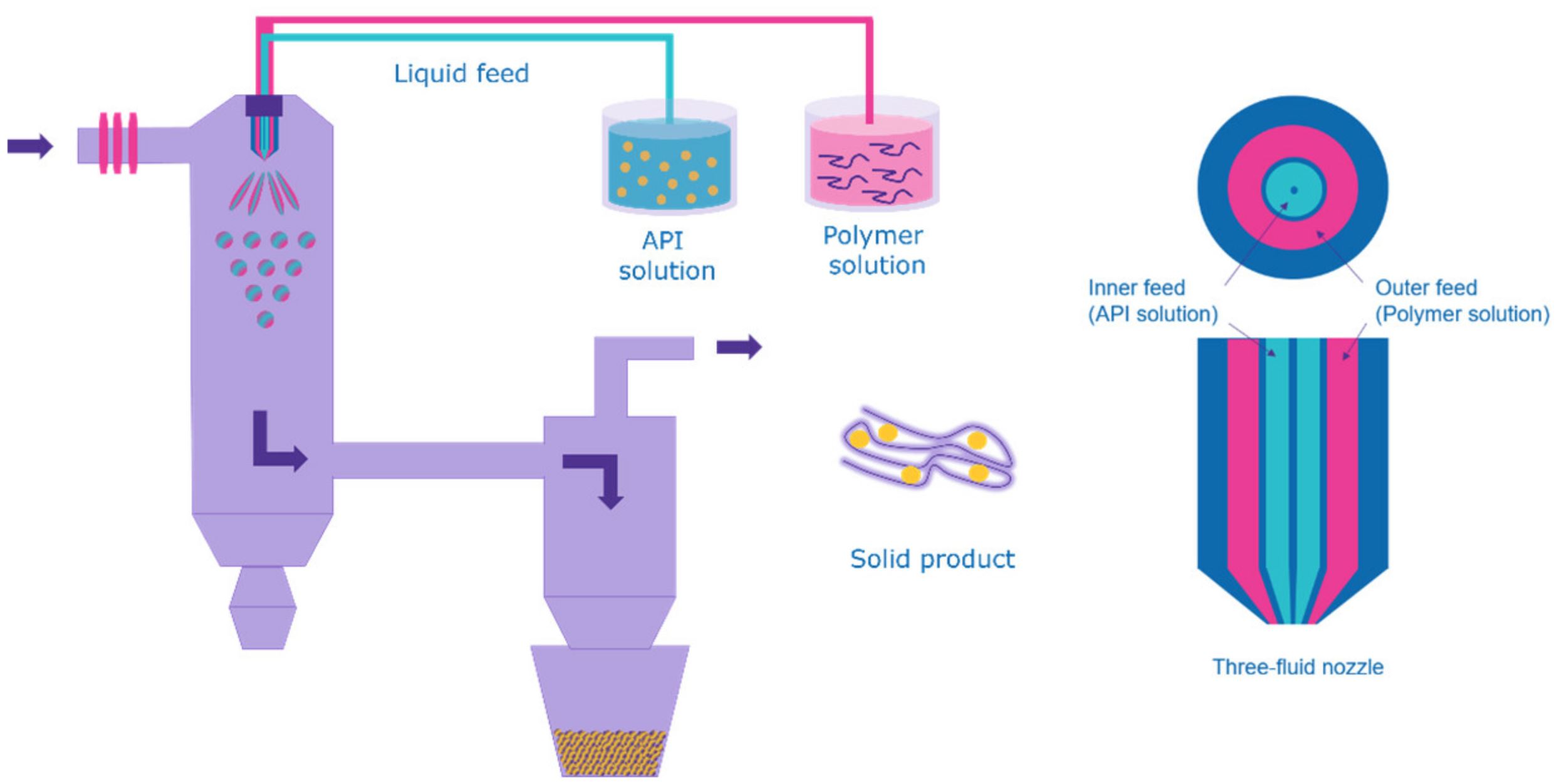
Classical spray drying (SD) processes are often limited due to low solubility of either the drug substance or required excipients. Preparations of two-phase systems like suspensions of colloids are often applied to overcome this issue. Four different types of atomizer technologies are used for most of the industrial SD applications: rotary atomizers, pressure nozzles, two-fluid nozzles and ultrasonic atomizers [3].
Hydrophilic polymers usually exhibit low solubility in organic solvents which is a critical drawback for applications like SD, where in most cases, the polymer is dissolved in a common solvent along with the drug compound to create a uniform solution which is then atomized into fine droplets for drying.
Attempts to enable the use of hydrophilic polymers for SD applications usually involve the creation of complex solvent systems and dedicated, often rather time-consuming preparation methods to create a common solvent system between low-soluble drug compounds and hydrophilic polymers. The resulting solid dispersions in many cases still indicate signs of crystallinity [4].
The use of three-fluid nozzle atomizers has been described for the development of dry powder combination products of theophylline and salbutamol for pulmonary delivery [5].
First attempts for the creation of solid dispersions via three-fluid nozzle systems were performed by Kauppinen et al. by investigating mannitol as a hydrophilic carrier for diazepam; instead of using one common solvent, two different solvents are used to prepare two different solutions to dissolve the carrier and API in separate vessels [6]. They are then pumped separately through two different channels and are combined at the tip of the nozzle, where they are rapidly mixed and atomized using pressurized gas added via the third channel.
The aim of this study is to evaluate a novel amorphization approach using a Multi Liquid Kinetic Technology (AMOR–MLKT approach). This process can serve as an alternative preparation concept to classical SD, enabling the use of hydrophilic polymers in spray drying applications while maintaining rapid preparation times and utilizing simple solvent systems. Immediate mixing of multiple solutions at the nozzle, combined with rapid drying kinetics assure a homogenous and stable ASD.
Various established polymers were evaluated during the study. Focus was also laid on a recently developed grade of polyvinyl alcohol (PVA). This grade differentiates from existing polymers due to its adapted hydrolysis degree (82%). It is of interest if adapted molecular properties leading to improved amphiphilicity enable the application of PVA for spray-drying approaches.
Download the full article as PDF here: Evaluation of a Three-Fluid Nozzle Spraying Process for Facilitating Spray Drying of Hydrophilic Polymers for the Creation of Amorphous Solid Dispersions
or read it here
Materials
Polyvinyl alcohol 3-82, (Parteck® MXP 3-82, EMPROVE® ESSENTIAL, Merck KGaA, Darmstadt, Germany), polyvinyl alcohol 4-88 (Parteck® MXP 4-88 EMPROVE® ESSENTIAL, Merck KGaA, Darmstadt, Germany), hydroxypropyl methylcellulose acetate succinate HPMC-AS (Affinisol™, Colorcon, Harleysville, PA, USA), polyvinylpyrrolidon PVP K30 (VWR Chemicals, Darmstadt, Germany), polyvinyl caprolactam polyvinyl acetate-polyethylene glycol grafted copolymer (Grafted copolymer, Soluplus® BASF, Ludwigshafen am Rhein, Germany), indomethacin (IND, Sigma Aldrich, St. Louis, MS, USA), ketoconazole (KET, Piramal, Mumbai, India), ritonavir (RIT, Aurobindo Pharma Ltd., Hyderabad, India), Norvir® (Abbott, Lake Bluff, IL, USA), simulated gastric fluid SGF (protocol by USP), fasted-state simulated intestinal fluid FaSSIF (Biorelevant, London, UK), methanol (Sigma Aldrich), acetone (Sigma Aldrich), ethanol (Sigma Aldrich), acetonitrile (Sigma Aldrich), Na3PO4*12 H2O (Sigma Aldrich), 0.1 M HCl (Sigma Aldrich), diisopropylamin (Sigma Aldrich), ammonium acetate (MilliporeSigma, Burlington, MA, USA).
Mueller, L.K.; Halstenberg, L.; Di Gallo, N.; Kipping, T. Evaluation of a Three-Fluid Nozzle Spraying Process for Facilitating Spray Drying of Hydrophilic Polymers for the Creation of Amorphous Solid Dispersions. Pharmaceutics 2023, 15, 2542. https://doi.org/10.3390/pharmaceutics15112542
Read more here Orally Disintegrating Tablets (ODTs) here:


