Premix technologies for drug delivery: manufacturing, applications, and opportunities in regulatory filing
Abstract
Active pharmaceutical ingredients (APIs) and excipients can be carefully combined in premix-based materials before being added to dosage forms, providing a flexible platform for the improvement of drug bioavailability, stability, and patient compliance. This is a promising and transformative approach in novel and generic product development, offering both the potential to overcome challenges in the delivery of complex APIs and viable solutions for bypassing patent hurdles in generic product filing. We discuss the different types of premixes; manufacturing technologies such as spray drying, hot melt extrusion, wet granulation, co-crystal, co-milling, co-precipitation; regulatory filing opportunities; and major bottlenecks in the use of premix materials in different aspects of pharmaceutical product development.
Highlights:
- Premix technology has applications in various aspects of novel and generic formulation development.
- We discuss major manufacturing technologies and characterize the techniques used for the preparation of pharmaceutical premixes.
- We highlight the scope for the regulatory filing of premixes and the current regulatory challenges facing the use of premix technology in pharmaceutical product development.
Introduction
The ever-evolving field of drug delivery is always in need of novel and cost-effective strategies for the delivery of complex molecules to their desired destination in a very precise manner. However, the new molecules that are coming to market mostly possess poor aqueous solubility.1 Therefore, solubility enhancement is sometimes required to achieve the desired pharmacological action at a minimal dose. Active molecule amorphization is a very popular approach to improve the solubility and bioavailability of poorly water-soluble drugs.2 Furthermore, newly launched drug products that contain novel molecules are generally patent protected in terms of the polymorphic form of the active molecule or a certain set of excipients that are crucial for delivering that drug.3 In such instances, the amorphization of the active molecule is the easiest and most promising way to bypass patenting hurdles. Nevertheless, the stabilization of amorphous forms of drug substances throughout their self-life is challenging.4 Such drug products have a high chance of failure through polymorphic conversion to a patented polymorphic form that will infringe the patent terms.5 This phenomenon is complex, can happen at any time of the product’s shelf-life, and is one of the biggest challenge for generic product filing through Para-IV certification.6
Sometimes, patent protection is obtained for specific excipients, which must be substituted with suitable excipients that have similar utility in order to bypass a patent. In addition, novel excipients that have tailormade properties are needed to meet the complex goals of many drug delivery strategies.7 The use of the complex form of the drug substance or of complex excipients provides an innovator company with a firm basis and confidence to believe that the product cannot be copied and that a stable source of revenue from the market can be guaranteed even after expiration of the patent terms. Eventually, the generic players will not be able to enter into the market so easily.8
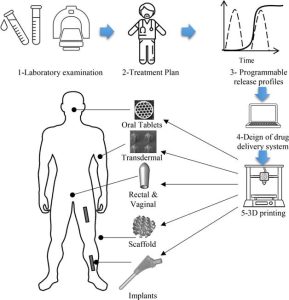
Premix-based drug-delivery approaches play a pivotal role in the development of novel drug products and can open up new horizons for both the innovator and the generic manufacturers.9 Premix-based approaches can help innovator organizations to develop a suitable formulation of complex molecules that conforms to desired bioavailability parameters.10 This approach helps the firm to obtain regulatory approval for the new drug and to introduce novel products into the market. On the other hand, a similar strategical approach can also help generic manufacturers to overcome the patent hurdles erected by the innovator firm in order to protect their novel product. In such instances, premix technologies can open up phenomenal opportunities; for example, where the crystalline form of an active pharmaceutical ingredient (API) is patent-protected, the use of premixes can be a very strong approach for patent bypassing and product filing in a way that does not infringe the patent. Thus, the same technology can provide financial benefits for both the innovator and the generic manufacturers.
A pharmaceutical premix can be defined as a mixture of individual native components that are intentionally mixed to impart or improve a certain quality through the mutual association of the participating components. In most cases, the mixture of components is physically bonded. The formation of premixes is not, however, limited to physical interactions only, and chemical interactions may also be involved in generating the desired product.11
API premixes are combinations containing one or more drugs along with a suitable vehicle. Carrier–carrier premixes of specific excipients may also be used to obtain a tailormade property. The various types of premixes that have been reported include drug–polymer, polymer–polymer, polymer–excipient, drug–drug, and excipient–excipient mixtures. The physical form of premix may be powder, granules, semi-solids or liquids.12 Powders or granules are free-flowing and homogenous. Homogenous suspensions or solutions are liquid forms that can be made from thixotropic gels or structured liquids. Such liquids can also serve as the base material for various dosage forms such as tablet, capsules or other pharmaceutical products.13 Premix technologies have been explored extensively for important applications beyond addressing solubility issues, such as tailormade excipient development, drug stability improvement, improvement of dosage uniformity, and taste masking.14
Although premixes are widely used to overcome multiple drug delivery challenges, they may also suffer from some novel difficulties. When combining different APIs in a premix formulation, their incompatible physical or chemical properties may cause stability issues, decreased efficacy, or unfavorable reactions. It can be difficult to ensure the compatibility of different APIs and substantial testing and formulation modifications may be necessary. A unit dosage form that contains several APIs may raise the possibility of drug interactions. Such combinations may change the pharmacokinetic or pharmacodynamics profile of a drug, which may lead to undesired therapeutic outcomes. It can be difficult to maintain consistent quality for all batches of a premix product, especially when working with different APIs that have different properties. Thus, strong quality control procedures and analytical techniques are essential in order to ensure that batch-to-batch consistency is monitored and maintained.
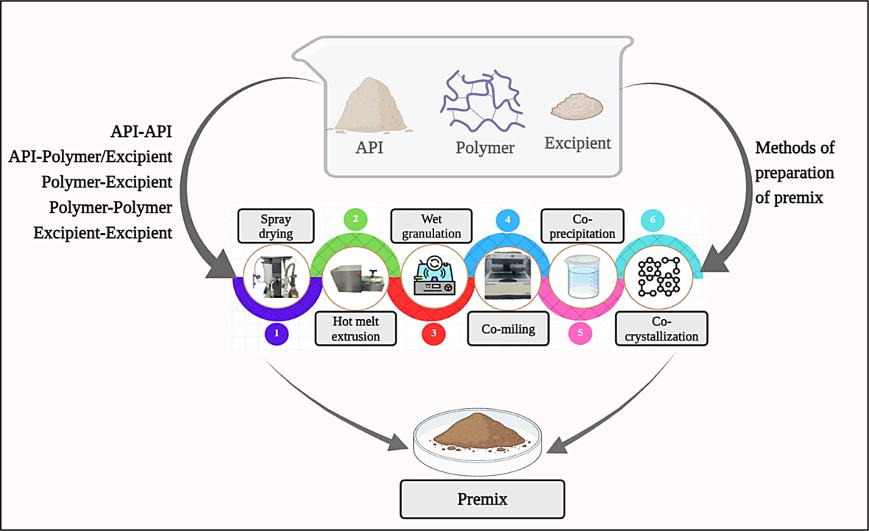
Current market projections predict a huge boost in the growth of premix-based API and excipient products in the coming years (Fig. 1).15 A major reason is the increasing complexity of the active molecules, which is introducing novel challenges regarding both delivery and toxicity issues. Innovative, sustainable, economic and green methods of manufacturing are also demanded to surmount the current challenges. Various manufacturing methods, including hot melting,16 spray drying,17 wet granulation,17 co-crystallization or co-amorphization, co-precipitation,19 co-milling,20 and many more techniques, are being explored as potential strategies the efficient and largescale manufacturing of premixes with tailored properties. Efficient characterization techniques and a set of recommended physicochemical analytical procedures are needed in order to obtain regulatory approval and to perfect the design of a drug master file (DMF).
This review article highlights the major technologies involved in developing premixes, detailing premix characterization techniques, the potential applications of and challenges facing premix technologies in pharmaceutical product development, and the current regulatory landscape for the application of premix technologies in novel formulations or generic products.
Read more here
Makka Krupali Ashokbhai, Lohare Rahul Sanjay, Sunil Kumar Sah, Subhadeep Roy, Santanu Kaity, Premix technologies for drug delivery: manufacturing, applications, and opportunities in regulatory filing, Drug Discovery Today, 2024, 104011, ISSN 1359-6446, https://doi.org/10.1016/j.drudis.2024.104011.
Read more interessting articles on “manufacturing technologies“ here:
- Better and greener: sustainable pharmaceutical manufacturing technologies for highly bioavailable solid dosage forms
- Oral drug delivery systems using core–shell structure additive manufacturing technologies
- Additive Manufacturing Technologies for Drug Delivery Applications