A weight of evidence review of the genotoxicity of titanium dioxide (TiO₂)
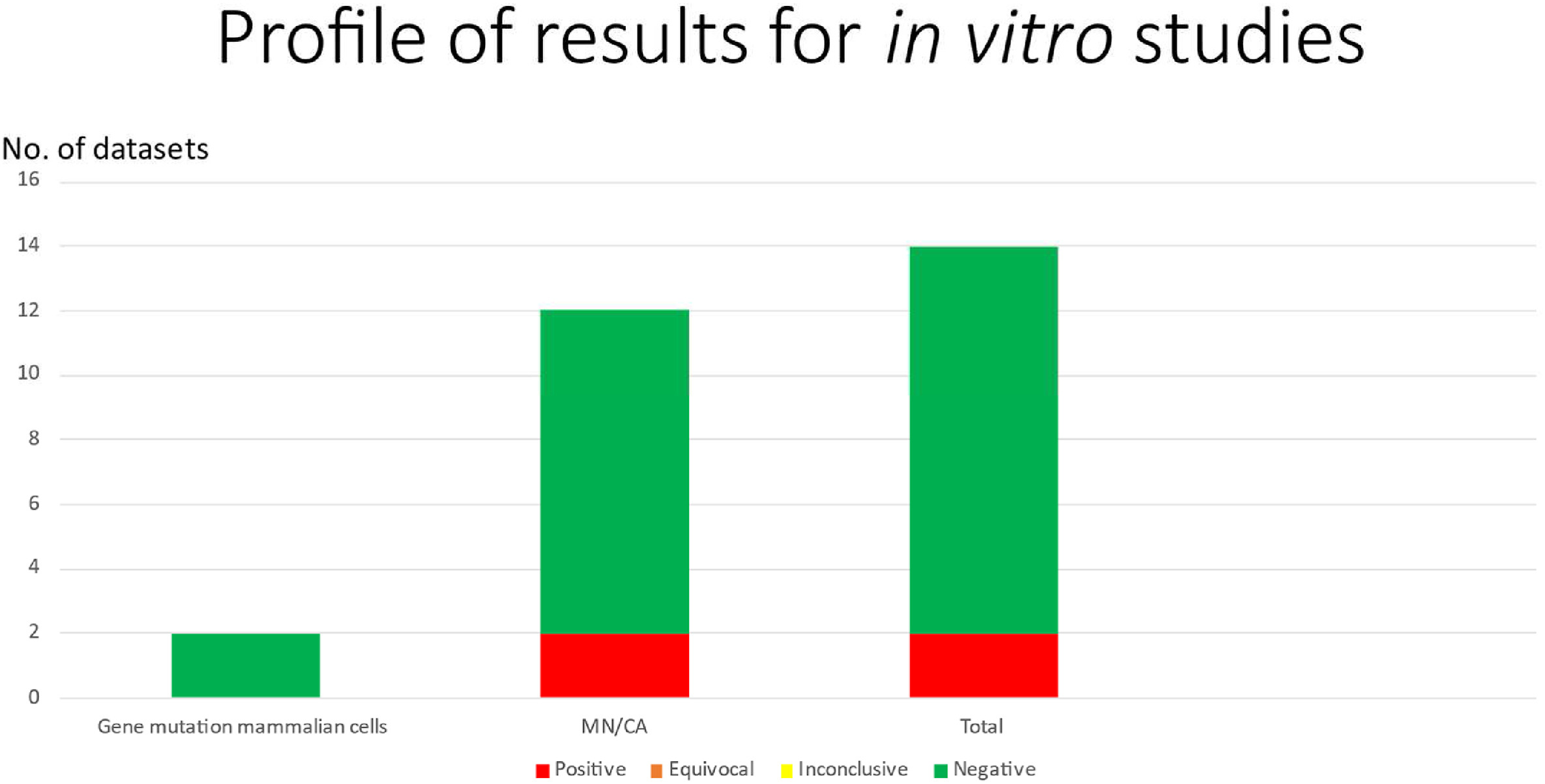
Titanium dioxide is a ubiquitous white material found in a diverse range of products from foods to sunscreens, as a pigment and thickener, amongst other uses. Titanium dioxide has been considered no longer safe for use in foods (nano and microparticles of E171) by the European Food Safety Authority (EFSA) due to concerns over genotoxicity. There are however, conflicting opinions regarding the safety of Titanium dioxide. In an attempt to clarify the situation, a comprehensive weight of evidence (WoE) assessment of the genotoxicity of titanium dioxide based on the available data was performed. A total of 192 datasets for endpoints and test systems considered the most relevant for identifying mutagenic and carcinogenic potential were reviewed and discussed for both reliability and relevance (by weight of evidence) and in the context of whether the physico-chemical properties of the particles had been characterised. The view of an independent panel of experts was that, of the 192 datasets identified, only 34 met the reliability and quality criteria for being most relevant in the evaluation of genotoxicity.
Highlights
- EFSA have recently banned titanium dioxide in foods due to concerns over genotoxicity
- A tiered weight of evidence analysis was performed on genotoxicity data for TiO2, according to relevance and reliability
- TiO2 was positive for chromosome damage mainly at levels where reactive oxygen or other cellular toxicity were prevalent.
- TiO2 was negative for point mutations in vivo, the panel noted more data would be required to make definitive conclusions.
Of these, 10 were positive (i.e. reported evidence that titanium dioxide was genotoxic), all of which were from studies of DNA strand breakage (comet assay) or chromosome damage (micronucleus or chromosome aberration assays). All the positive findings were associated with high cytotoxicity, oxidative stress, inflammation, apoptosis, necrosis, or combinations of these. Considering that DNA and chromosome breakage can be secondary to physiological stress, it is highly likely that the observed genotoxic effects of titanium dioxide, including those with nanoparticles, are secondary to physiological stress. Consistent with this finding, there were no positive results from the in vitro and in vivo gene mutation studies evaluated, although it should be noted that to definitively conclude a lack of mutagenicity, more robust in vitro and in vivo gene mutation studies would be useful.
Existing evidence does not therefore support a direct DNA damaging mechanism for titanium dioxide (nano and other forms).
Download the research paper as PDF (pre-proof): A weight of evidence review of the genotoxicity of titanium dioxide
or continue here with genotoxicity of titanium dioxide
David Kirkland, Marilyn J. Aardema, Rüdiger V. Battersby, Carol Beevers, Karin Burnett, Arne Burzlaff, Andreas Czich, E. Maria Donner, Paul Fowler, Helinor J. Johnston, Harald F. Krug, Stefan Pfuhler, Leon F. Stankowski,
A weight of evidence review of the genotoxicity of titanium dioxide (TiO₂),
Regulatory Toxicology and Pharmacology, 2022, 105263, ISSN 0273-2300,
https://doi.org/10.1016/j.yrtph.2022.105263.
See coating solutions without TiO2 :


