Integrating pressure sensor control into semi-solid extrusion 3D printing to optimize medicine manufacturing
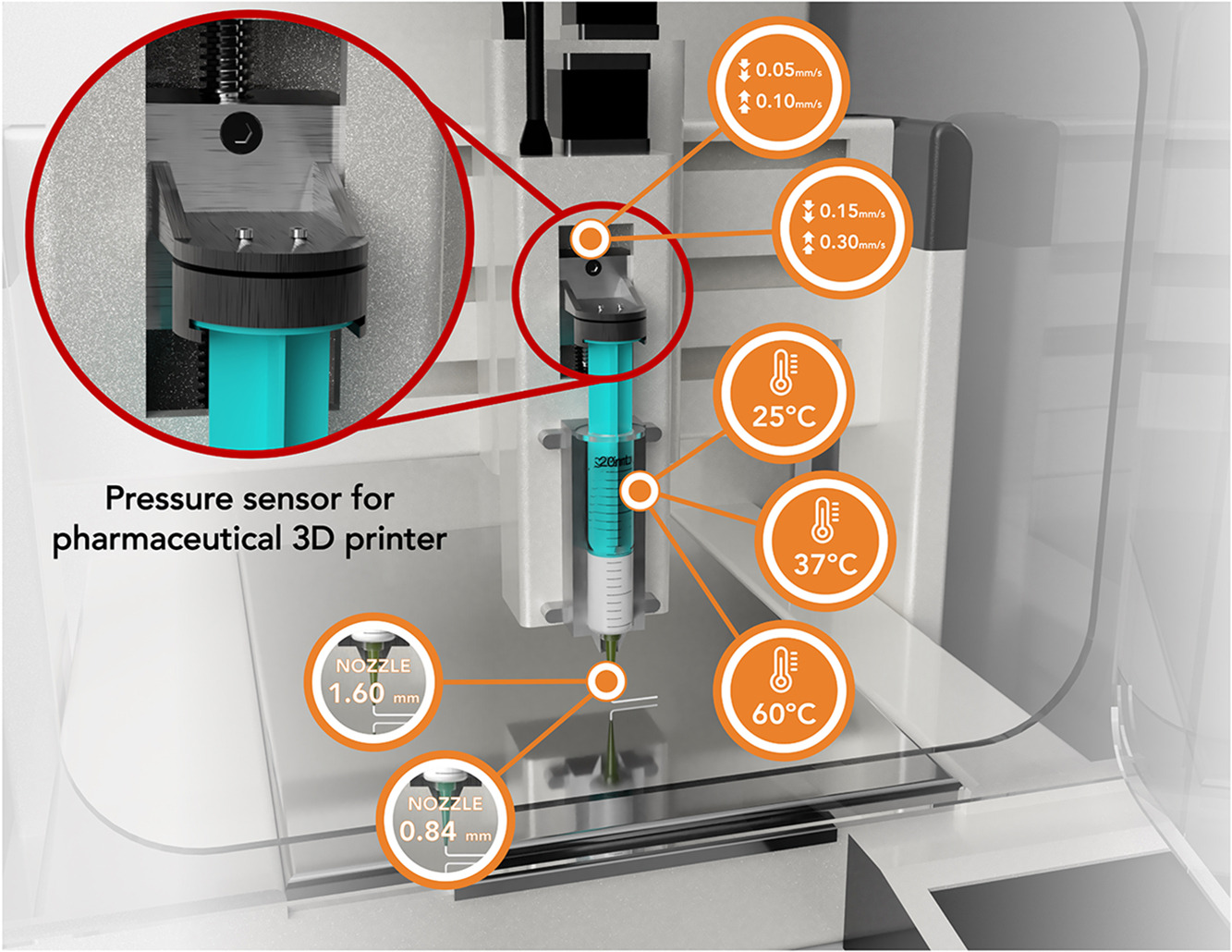
Semi-solid extrusion (SSE) is a three-dimensional printing (3DP) process that involves the extrusion of a gel or paste-like material via a syringe-based printhead to create the desired object. In pharmaceuticals, SSE 3DP has already been used to manufacture formulations for human clinical studies. To further support its clinical adoption, the use of a pressure sensor may provide information on the printability of the feedstock material in situ and under the exact printing conditions for quality control purposes. This study aimed to integrate a pressure sensor in an SSE pharmaceutical 3D printer for both material characterization and as a process analytical technology (PAT) to monitor the printing process. In this study, three materials of different consistency were tested (soft vaseline, gel-like mass and paste-like mass) under 12 different conditions, by changing flow rate, temperature, or nozzle diameter.
Highlights
- In this study, a novel printhead with an integrated pressure sensor was developed.
- In-situ material characterization and monitoring of process parameters was achieved.
- The technology reported herein supports the deployment of SSE printers in clinics.
The use of a pressure sensor allowed, for the first time, the characterization of rheological properties of the inks, which exhibited temperature-dependent, plastic and viscoelastic behaviours. Controlling critical material attributes and 3D printing process parameters may allow a quality by design (QbD) approach to facilitate a high-fidelity 3D printing process critical for the future of personalized medicine.
Download the research paper as PDF (pre-proof): Integrating pressure sensor control into semi-solid extrusion 3D printing to optimize medicine manufacturing
or continue here with Integrating pressure sensor control into semi-solid extrusion 3D printing
Materials used in the study beside others:
Chewable gel-based formulation was provided by FabRx Ltd. (United Kingdom). L-Isoleucine was purchased from Nutricia (Netherlands). Vaseline soft, polyvinylpyrrolidone (PVP) and oral banana essence were provided by Acofarma. Ac-Di-Sol® (Croscarmellose Sodium) was provided by FMC Corporation (Philadelphia, PA, USA). Lactose monohydrate was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Eduardo Díaz-Torres, Lucía Rodríguez-Pombo, Jun Jie Ong, Abdul W. Basit, Ana Santoveña-Estévez, José B. Fariña, Carmen Alvarez-Lorenzo, Alvaro Goyanes,
Integrating pressure sensor control into semi-solid extrusion 3D printing to optimize medicine manufacturing,
International Journal of Pharmaceutics: X,2022, 100133, ISSN 2590-1567,
https://doi.org/10.1016/j.ijpx.2022.100133.

