Technological advances and challenges for exploring attribute transmission in tablet development by high shear wet granulation
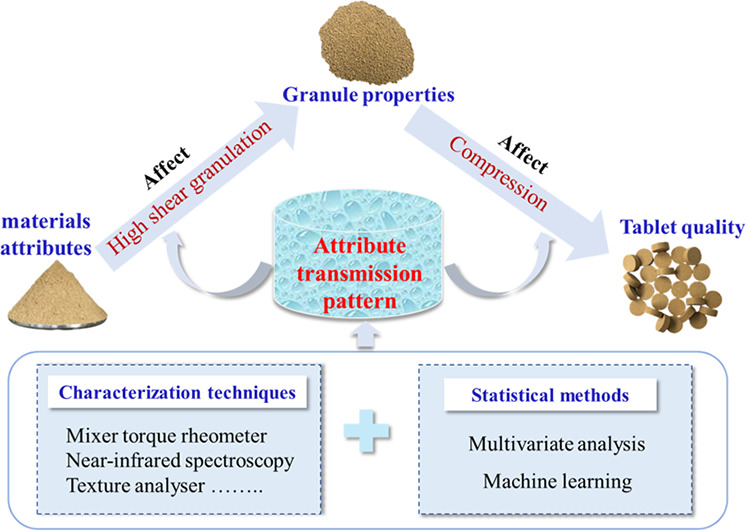
High shear wet granulation and tableting (HSWG-T) are complex pharmaceutical processes, which makes tablet quality be influenced by many variables including raw material attributes, process variables, and intermediate granule properties. To achieve the desired final quality of tablets and improve the production efficiency, it is necessary and urgent to comprehensively understand the attribute transmission pattern in HSWG-T. However, this aspect seems to be unexpectedly ignored in terms of reviews published so far. Therefore, it is the major focus in this review and was summarized and discussed based on the quality by design (QbD) concept. Additionally, the applications of characterization techniques and current multivariate analysis models in the study of attribute transmission pattern were also reviewed. As expected, there are some certain attribute transmission patterns in HSWG-T. Moreover, some attributes are highly correlated, and their interactions are also important for the results. Therefore, this review is beneficial for QbD in HSWG-T. It can also provide some guidance for further studies.
Introduction
Nowadays, tablets are the most popular solid oral dosage form in drug manufacturing, accounting for 20% of all formulations in the 2020 Chinese Pharmacopoeia and nearly 70% of the drugs market [1]. There are three basic tablet production methods, namely: direct compaction (DC), wet granulation, and dry granulation. DC technique is considered the preferred tablet production method due to its simpleness, time and cost effectiveness, and elimination of moisture and heat effects [2]. However, in order to obtain uniform die filling and produce tablets with sufficient quality, it is often necessary to improve the compaction and flow properties of the material by converting fine powders into large agglomerate through wet or dry granulation process. Granulation is a considerable process employed in the pharmaceutical industry to produce a sequence of solid dosage forms, principally in the preparation of tablets and capsules [[3], [4], [5]]. A recently published survey of commercial solid oral dosage forms revealed that wet granulation accounted for 40% of all tablet formulations [6]. Currently, wet granulation technologies mainly include (i) high shear wet granulation (HSWG), (ii) fluid-bed granulation, (iii) twin-screw granulation, and (iv) melt fluid-bed granulation [6,7]. Among the multiple granulation methods, HSWG is generally used in the tableting process attributed to the advantages of using less binder, short processing time, the formation of the uniform particle size distribution (PSD) of granules, and dense granules suitable for tablet compression, etc., particularly at high drug loads [[8], [9], [10]]. Meanwhile, HSWG can be described as the interaction between a binder or wetting agent and a drug powder under high shear. Classically, the HSWG process needs to go through three complex stages to form granules, namely, (i) wetting and nucleation, (ii) growth and consolidation, and (iii) attrition and breakage of granules [11].
High shear wet granulation and tableting (HSWG-T) process involves a series of operation units, and primary interfering factors in the process are described by a fishbone diagram in Fig. 1 [3,12]. This means that the quality of tablets prepared by the HSWGT depends largely on multiple variables, which increases the difficulty of quality control. For example, related studies have reported that the moisture content of raw materials affected quality attributes of granules, such as flowability and compactibility [13,14]. In addition, granule density and strength also affect re-arrangement, deformation, and fragmentation of granules during tablet compression, which further affects the tensile strength, dissolution capacity, and disintegration time of tablets [[15], [16], [17], [18]]. In this review, attribute transmission refers to the property transfer from raw materials to granules and granules to tablets during the HSWG-T process. For the above reasons, attribute transmission should be analyzed. Meanwhile, developing an effective quality control strategy to obtain acceptable tablets.
Interestingly, attribute transmission in the HSWG-T process is consistent with the Quality by Design (QbD) concept. QbD was introduced by Dr. Joseph M. Juran in 1992 [19]. It highlights a shift in the drug quality control system from only relying on final product inspection in the past to quality control in the product design, manufacturing process, and research phases [20]. Moreover, the overall framework and implementation details of QbD in drug manufacturing and development were described in four guidelines issued by International Conference on Harmonization (ICH), including ICH Q8, Q9, Q10, and Q11 [1]. Thus, identifying the correlation of the critical material attributes (CMAs) of raw materials or critical process parameters (CPPs) and the intermediate granule properties to the critical quality attributes (CQAs) of final tablet products is critical to improving the quality and stability of products and the controllability of production processes [21,22]. In recent years, due to the promotion and application of QbD concept, modeling and simulation technology, and process analysis technology (PAT) in the pharmaceutical industry, researchers have gained an in-depth understanding of attribute transmission in the HSWG-T process. For example, many characterization techniques including mixer torque rheometer (MTR), texture analyser (TA), near-infrared (NIR) spectroscopy, etc., provide technical support for effective analysis of the attribute transmission pattern. These techniques allow the monitoring of raw materials attributes, intermediate granule attributes, final tablet product quality, and other high-risk variables. Additionally, since using a large amount of information to create models allows numerical predictions and the establishment of design spaces, multivariate analysis (MVA) models have certainly attracted the attention of many researchers. For example, Dai et al. proposed a process model for DC of pharmaceutical powders and successfully predicted tablet hardness and tensile strength from raw material properties [23].
At present, there are some reviews focusing on the effects of formulation design and process parameters on HSWG [3,24,25]. However, the attribute transmission pattern among material, granules, and tablets in the HSWG-T process is yet to be clarified, especially in terms of the influences of granule attributes on tablet quality. Therefore, in this review, we first provide the latest advances and development of attribute transmission in the HSWG-T process. Second, some common characterization techniques and MVA models used in the study of attribute transmission pattern are further reviewed. Finally, the future challenges for studying attribute transmission during the HSWG-T process are proposed with the aim of providing more information references for further studies.
Read more
LiangFeng Wang, LiJie Zhao, YanLong Hong, Lan Shen, Xiao Lin, Technological advances and challenges for exploring attribute transmission in tablet development by high shear wet granulation, Powder Technology, 2023, 118402, ISSN 0032-5910, https://doi.org/10.1016/j.powtec.2023.118402.

