Effect of a superdisintegrant on disintegration of orally disintegrating tablets determined by simulated wetting test and in vitro disintegration test
Abstract
1. Introduction
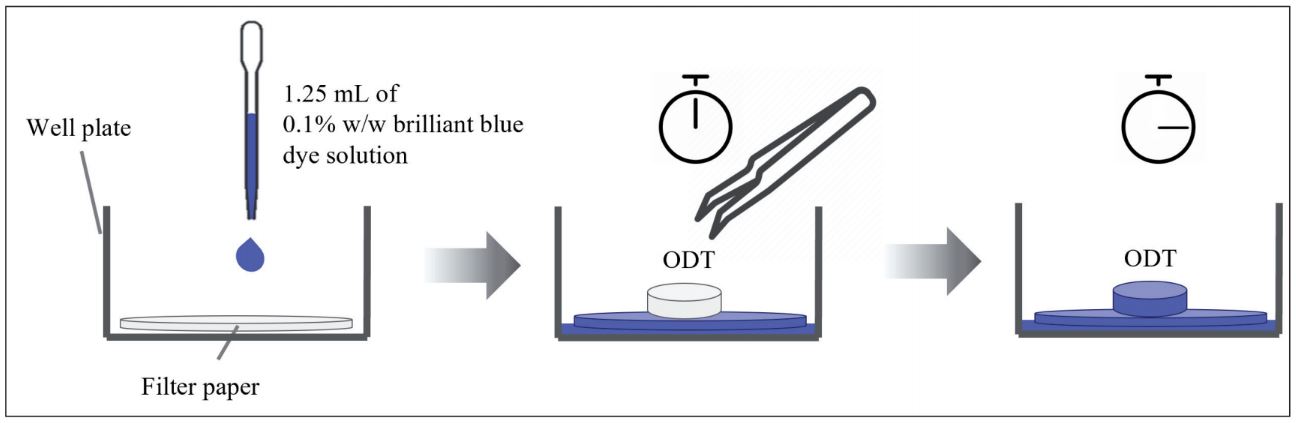
Several types of dosage forms have been developed in recent years to fulfill a variety of patient demands, especially to improve compliance. Orally disintegrating tablets (ODTs) are one of these items that are specially designed for patients who have swallowing difficulties with conventional dosage forms. ODTs may be made using a variety of technologies. Molding, lyophilization, and direct compression are the most often used preparation processes. Direct compression is a typical tablet production procedure for ODTs. It is frequently preferred over alternative ODT production procedures because it leverages current high-speed tablet press equipment and common excipients (Trisopon et al. 2021). A direct-compression formulation offers better physical qualities than conventional approaches, which may reduce the requirement for specific packaging such as blister containers. Direct-compression ODT formulations often contain high amounts of a superdisintegrant, including sodium starch glycolate, croscarmellose sodium, and crospovidone, to induce fast disintegration (Ahmad 2018; Desai et al. 2016). Thus, selecting the proper superdisintegrant is crucial when designing an ODT formulation for direct compression. The disintegration time of a product is the most important criteria in classifying it as an ODT. According to the US FDA recommendation, an ODT should dissolve quickly, with an in vitro disintegration time of 30 s or less using the United States Pharmacopeia (USP) disintegration technique or an alternative (Food and Drug Administration, 2008). The disintegration test recommended by the USP requires 900 mL of water. Recent study on ODTs indicated that this volume did not accurately mimic the oral environment and that a smaller volume of water that closely represented the oral environment was necessary (Hooper et al. 2016). Several alternative disintegration tests have been proposed (Abay and Ugurlu 2015; Hooper et al. 2016; Chinwala 2020; Ghourichay et al. 2021). Among these methods, the wetting test has received a lot of interest in characterization of ODTs due to its convenience and simplicity. However, it takes some time for the dye solution to diffuse and cover the entire surface of the tablet. In addition to the wetting test, other alternative methods for determining in vitro disintegration time have been proposed in recent years, including modified USP dissolution apparatus II (Bi et al. 1996), charge coupled device camera (Morita et al. 2002), shaking bath (Fu et al. 2006), rotary shaft (Narazaki et al. 2004), in vitro disintegration test by medium dripping (Hoashi et al. 2013), and texture analyzer (Scheuerle et al. 2015), among others. The purpose of this study was to investigate the effect of several superdisintegrants, i.e., sodium starch glycolate, croscarmellose sodium, and crospovidone on the disintegration of ODTs using two approaches, namely simulated wetting and in vitro disintegration tests. The simulated wetting test was slightly modified from the method proposed by Park and colleagues (Park et al. 2008). The concept behind this technique is to place a tablet on a colored, wet filter paper and then record the color diffusion on the tablet until it is completely covered. The in vitro disintegration test described by Hoashi et al. (2013) was adopted in this investigation with minor modifications since it requires a simple equipment setup and can simulate the physiological conditions in the mouth. The in vitro disintegration test method involves dripping water on top of the mesh-encased tablet. The time required for the upper mesh to completely touch the lower mesh is then recorded. The relationship between simulated wetting time and in vitro disintegration time of these ODTs was also examined.
3.1. Materials
Mannitol (lot number 302004308) was obtained from Shandong Tianli Pharmaceutical Co., Ltd., China. Spray dried lactose monohydrate (Supertab®11SD, lot number 23034009) and sodium starch glycolate (Primojel®, lot number 10519TW) were purchased from DMV-Fonterra Excipients GmbH & Co., Germany. Microcrystalline cellulose (Comprecel® M101D+, lot number C2006037) and croscarmellose sodium (Disolcel®, lot number D02003103) were obtained from Mingtai Chemical Co., Ltd., China. Crospovidone (Polyplasdone XL®, lot number 0002434812) and polyvinyl pyrrolidone K-30 (PVP K-30, lot number 002377911) were received from Ashland Chemical Inc., USA. Magnesium stearate (Kemilub EM-F-V®) was obtained from Italmatch Chemicals, Spain.
L. Sutthapitaksakul, K. Thanawuth, P. Sriamornsak, Effect of a superdisintegrant on disintegration of orally disintegrating tablets determined by simulated wetting test and in vitro disintegration test, Pharmazie 77: 287-290 (2022)
doi: 10.1691/ph.2022.2015

