Factors That Influence Sustained Release from Hot-Melt Extrudates
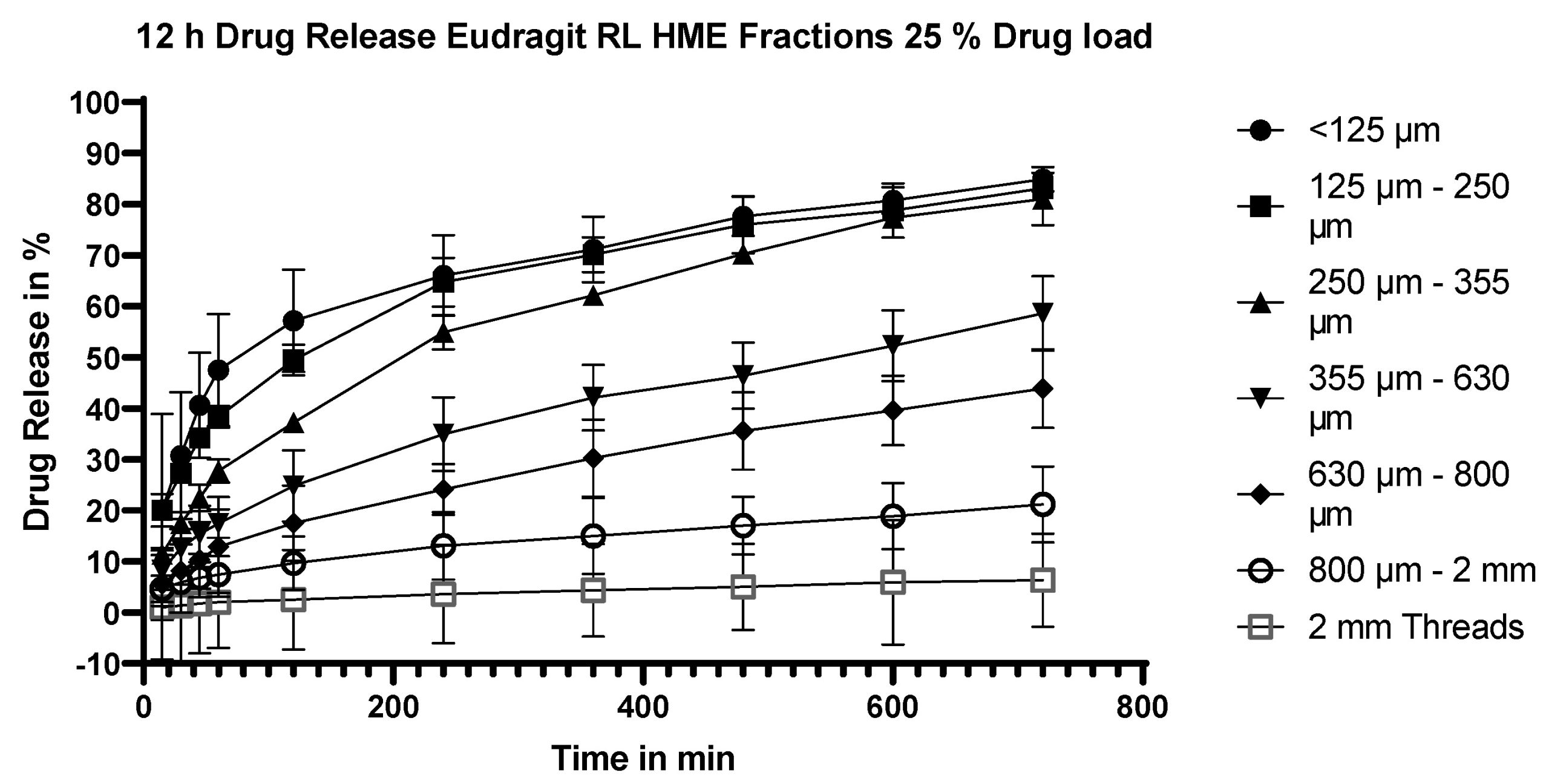
Hot-melt extrusion is a well-established tool in the pharmaceutical industry, mostly implemented to increase the solubility of poorly soluble drugs. A less frequent application of this technique is to obtain formulations with extended release. This study investigated the influence of polymer choice, drug loading, milling and hydrodynamics on the release of a model drug, flurbiprofen, from sustained-release hot-melt extrudates with Eudragit polymers. The choice of polymer and degree of particle size reduction of the extrudate by milling were the two key influences on the release profile: the percentage release after 12 h varied from 6% (2 mm threads) to 84% (particle size <125 µm) for Eudragit RL extrudates vs. 4.5 to 62% for the corresponding Eudragit RS extrudates. By contrast, the release profile was largely independent of drug loading and robust to hydrodynamics in the dissolution vessel. Thus, hot-melt extrusion offers the ability to tailor the release of the API to the therapeutic indication through a combination of particle size and polymer choice while providing robustness over a wide range of hydrodynamic conditions.
1. Introduction
2.1. Materials
Download the full article as PDF here: Factors That Influence Sustained Release from Hot-Melt Extrudates
or read it here
Mansuroglu, Y.; Dressman, J. Factors That Influence Sustained Release from Hot-Melt Extrudates. Pharmaceutics 2023, 15, 1996.
https://doi.org/10.3390/pharmaceutics15071996

