Tamoxifen-Loaded Eudragit Nanoparticles: Quality by Design Approach for Optimization of Nanoparticles as Delivery System
Abstract
Nanoparticles have numerous applications as drug carriers in drug delivery. The aim of the study was to produce tamoxifen nanoparticles with a defined size and higher encapsulation for efficient tissue uptake with controlled drug release. The quality by design approach was utilized to produce tamoxifen-loaded Eudragit nanoparticles by identifying the significant process variables using the nanoprecipitation method. The process variables (amount of drug, polymer, and surfactant) were altered to analyze the influence on particle size (PS), % encapsulation efficiency (EE). The results showed that the drug and polymer individually as well as collectively have an impact on PS, while the surfactant has no impact on the PS. The %EE was influenced by the surfactant individually and in interaction with the drug. The linear regression model was endorsed to fit the data showing high R2 values (PS, 0.9146, %EE, 0.9070) and low p values (PS, 0.0004, EE, 0.0005). The PS and EE were confirmed to be 178 nm and 90%, respectively. The nanoparticles were of spherical shape, as confirmed by SEM and TEM. The FTIR confirmed the absence of any incompatibility among the ingredients. The TGA confirmed that the NPs were thermally stable. The in vitro release predicted that the drug release followed Higuchi model.
Introduction
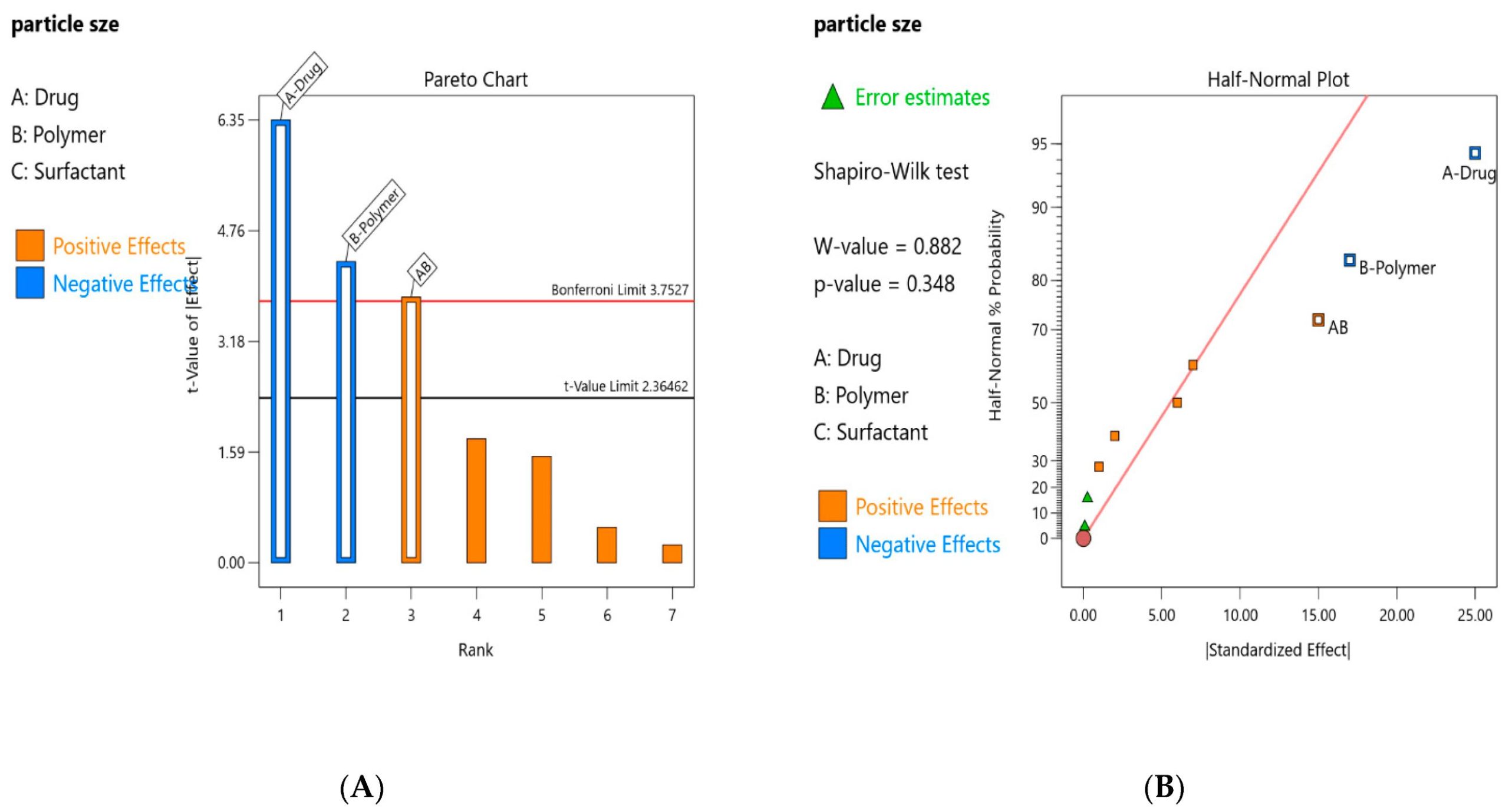
For multimodal treatment, the nanotechnology-based treatment approaches showed promising results, due to their high potential to improve the delivery of chemotherapeutic agents to cancerous cells while minimizing its distribution to normal cells [1,2,3,4,5]. The limitations related to chemotherapy can be overcome by employing the strategies of the nanomedicine to the anticancer drug formulations by using several nanocarriers [6,7,8,9]. For nano and microcarriers, polymers are the most reliable materials [10]. Apart from the route of administration, particle size, shape and surface charge and functional group play a key role in the controlled uptake of polymeric nanoparticles [11]. It is essential to control the modification of particle properties, depending on the target cell, to deliver the drug to the desired target site. For the development of these tailored particles, it is crucial to understand the process of preparation and the variables that influence the final product. This allows the management and control of production [12].
The production method still faces multiple challenges, such as control and replication of the required nanoparticles, which make it difficult to generate high-quality PNP (polymeric nanoparticles) despite major significant advances in laboratory-scale PNP preparation. PNP preparation procedures are frequently multi-step bulk processes with numerous affecting variables, making the preparation quite difficult. The use of quality by design (QbD) is crucial and requested by ICH to guarantee the final quality of the product early in the development of the technique and to understand as early as is feasible what factors influence the process. Within this frame of reference, designing Eudragit NPs of TAM that have a small particle size and high encapsulation efficiency optimized by factorial design is notable [13].
Quality by design is an analytical approach used in the development of pharmaceuticals. It entails evaluating and understanding the manufacturing and formulation processes. The objective is to develop a controlled procedure that ensures the product’s quality. Different methods and tools to implement QbD in practice are described in the ICH guideline Q8(R2), including “multivariate experiments”, “statistical process control methods”, also known as design of experiments, and a “risk-based control strategy [14]”.
The present study shows the application of the QbD by development of tamoxifen loaded Eudragit nanoparticles. The Eudragit RL is cationic copolymer derived from acrylic acid and methacrylic acid esters with quaternary ammonium groups [15]. Nowadays, the Eudragit RL and RS polymers are the pharmaceutical’s industry preferred choice for sustained drug release profiles due to their pH-independent swelling characteristics [16,17]. Various factors including the concentration of the drug, polymer, surfactant and solvent highly influence the particle size and %EE. When these factors are controlled, the nanoparticle of the desired profile can be prepared [18].
Tamoxifen is a non-steroidal antiestrogen and a selective estrogen receptor modulator. It has been clinically used for more than 20 years for the antiestrogenic therapy of malignancy or advanced breast cancer [19]. It has been used as an additional therapy following post-menopausal cancer and primary treatment of early-stage breast cancer [20]. Tamoxifen showed 20–30% oral bioavailability due to an extensive intestinal and hepatic first-pass effect, so the requirement of the dose is increased along with chronic (long-term) duration of therapy [21,22,23,24,25]. Tamoxifen is associated with multifocal hepatic fatty infiltration, toxic hepatitis and hepatic necrosis and cirrhosis [26]. Tamoxifen is also associated with increased risk of endometrial cancer, which is mainly due to its long-term treatment and dose accumulation [24]. Therefore, an alternate therapy is required for optimal chronic administration of tamoxifen with enhanced bioavailability and reduced adverse effects (hepatotoxicity).
This paper presents the utilization of QbD analysis and optimization for the development of tamoxifen-loaded Eudragit nanoparticles. In this study, the effects of multiple factors are being studied. The factorial experimental design is ideal for this type of study. The mathematical model included in this study evaluates the linear effects of the multiple factors as well as the effects of their interactions [27]. Compared with OFAT, factorial design provides more information and finds optimal conditions faster than OFAT experiments. OFAT experimental design cannot detect if the effect of one factor is different for different levels of another factor. To detect such interactions, factorial design is obligatory [28,29]. Identifying the process variables that have an impact on the finished product will be enough to change its attributes in a targeted manner, which is crucial for guided optimization. The study was divided into five phases.
The planning phase includes the selection of responses (outcome parameters) and identifying and assessing process variables that can affect the characteristics of Eudragit-NP.
- The screening phase includes the screening of the most promising variables to identify relevant process parameters and the first strategy for optimization using the screening findings.
- The optimization phase includes controlled modification of final product quality by altering the most impactful parameters within the RSM (response surface methodology)
- The verification phase includes prediction and confirmation of the ideal process variables.
Download the full article as PDF here Tamoxifen-Loaded Eudragit Nanoparticles: Quality by Design Approach for Optimization of Nanoparticles as Delivery System
or read it here
Materials
Tamoxifen citrate (purity 99.9%), purchased from Huzhou Zhanwang Pharma Co., Ltd., Huzhou, China, Eudragit RS 100 (Evonik, Essen, Germany); Acetonitrile (purity > 99.9%) was purchased from Fisher Scientific PVT. U.K. Ltd. (Loughborough, UK), Methanol (purity > 99.9%), Sodium dodecyl sulphate, Dialysis Tubing-Visking, (MWCO; 12–14 kDa); (Dia = 27/32″–21.5 mm); (Size 6 Inf. 30 M) (Sigma-Aldrich), distilled water [Millipore ultrapure water sys (Milford, CT, USA)].
Khattak, M.A.; Iqbal, Z.; Nasir, F.; Neau, S.H.; Khan, S.I.; Hidayatullah, T.; Pervez, S.; Sakhi, M.; Zainab, S.R.; Gohar, S.; et al. Tamoxifen-Loaded Eudragit Nanoparticles: Quality by Design Approach for Optimization of Nanoparticles as Delivery System. Pharmaceutics 2023, 15, 2373. https://doi.org/10.3390/pharmaceutics15102373
See the webinar:
“Enhanced Stability and Bioavailability of Poorly Soluble APIs“,
18 October 2023:
Get more information & register here for free:


