Silicon Oxycarbide Porous Particles and Film Coating as Strategies for Tenofovir Controlled Release in Vaginal Tablets for HIV Prevention
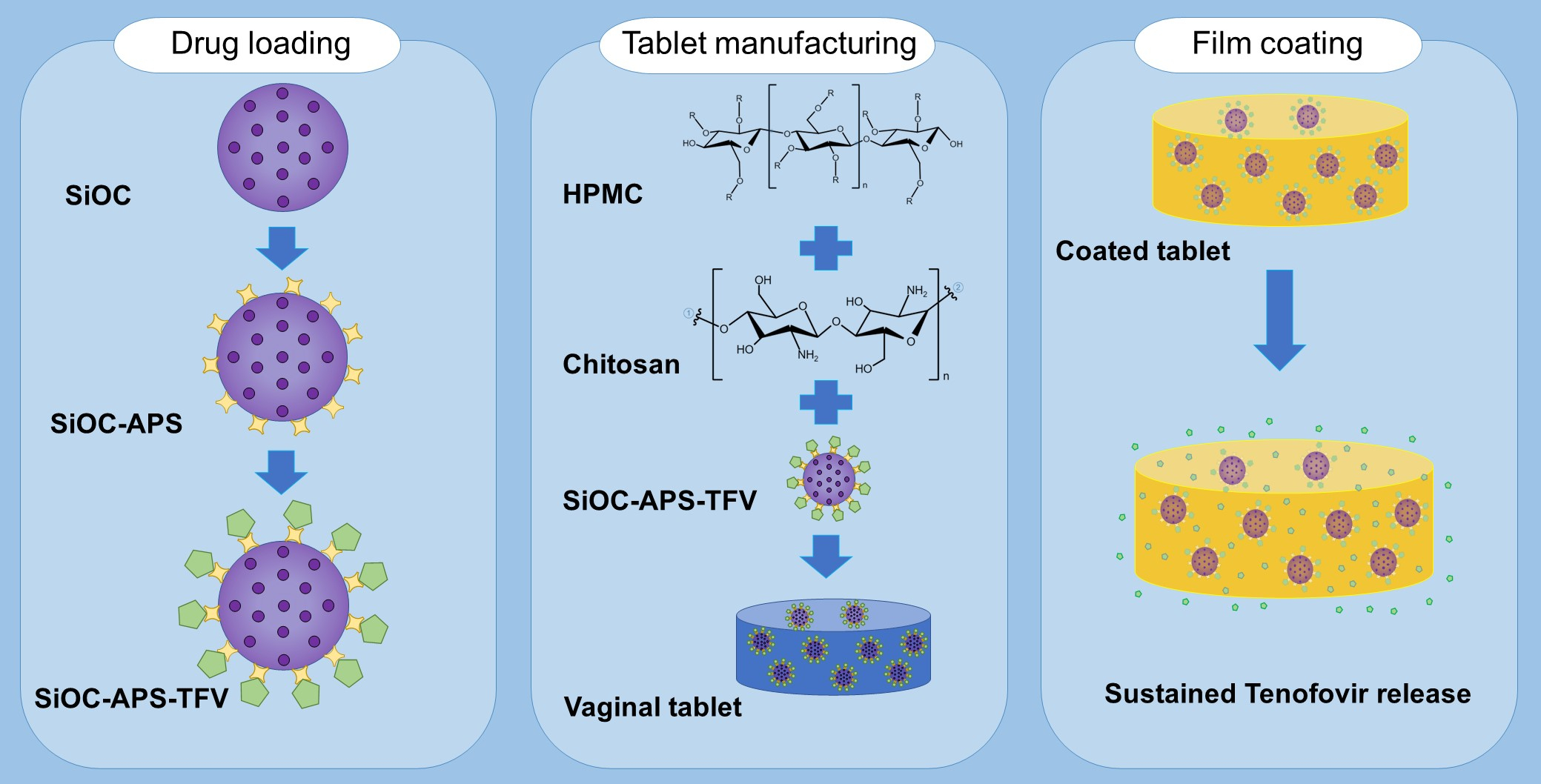
Sustained release of antiretroviral drugs is currently the most encouraging strategy for the prevention of the sexual transmission of HIV. Vaginal tablets based on hydrophilic gelling polymers are an interesting dosage form for this purpose, since they can be developed to modify the release of the drug depending on the tablet swelling. Tenofovir is a drug with proven activity in the prevention of HIV-1 infection, and it is possible to have it loaded in the surface of γ-aminopropyl trimethoxy silane-functionalized oxycarbide particles. These particles can be incorporated into the tablets, thus providing a sustained release of the drug. Moreover, the presence of the particles modifies the microstructure of the gel formed, as observed in scanning electron microscopy and Hg porosimetry studies, resulting into a gel with a narrow pore size distribution between 10 and 100 µm. This implies a lower volume of fluid incorporated into the gel during swelling studies, and therefore improved mucoadhesion times in ex vivo test. The coating of the formulations with Eudragit® RS modifies the swelling behavior of the tablets, which not only is decreased in magnitude but also extended in time, and as consequence the drug release is also prolonged for up to 7 days.
Download the full article as PDF here Silicon Oxycarbide Porous Particles and Film Coating as Strategies for Tenofovir Controlled Release in Vaginal Tablets for HIV Prevention
or read it here
Materials
Triethoxysilane (TREOS, 99%), isopropanol (iPrOH, 99%), HCl (35%), and NH4OH (28%) were acquired from Merck (Darmstadt, Germany). Hydroxyl terminated polydimethyl siloxane (PDMS, M = 1700 g·mol−1) and γ-aminopropyl trimethoxy silane (APS, 98%) were supplied by ABCR (Karlsruhe, Germany) and Gelest (Morrisville, PA, USA), respectively. Chitosan (MW = 105 g/mol, deacetylation degree = 97% and viscosity = 92 mPa·s [21]) was supplied by Nessler (Madrid, Spain) and hydroxypropyl methylcellulose Methocel® K 100 M (HPMC, MW = 72 × 104 g/mol) was a kind gift from Colorcon Ltd. (Kent, UK). Kollidon® 30 (polyvinyl pyrrolidone K30, PVP) and magnesium stearate PRS-CODEX (MgSt) were supplied by BASF (Ludwingshafen, Germany) and Panreac (Barcelona, Spain), respectively. Tenofovir (TFV) was acquired from Carbosynth Limited (Compton, UK). Eudragit® RS (ERS, MW = 407.932 g/mol) was kindly supplied by Evonik (Essen, Germany). Triethyl citrate (TEC) and acetone were acquired from Sigma-Aldrich® (St. Louis, MO, USA) and Panreac (Barcelona, Spain), respectively.
All other reagents in this study were of analytical grade and used without further purification. Demineralized water was used in all cases.
Martín-Illana, A.; Cazorla-Luna, R.; Notario-Pérez, F.; Ruiz-Caro, R.; Rubio, J.; Tamayo, A.; Veiga, M.D. Silicon Oxycarbide Porous Particles and Film Coating as Strategies for Tenofovir Controlled Release in Vaginal Tablets for HIV Prevention. Pharmaceutics 2022, 14, 1567. https://doi.org/10.3390/pharmaceutics14081567

