A vascularized in vivo melanoma model suitable for metastasis research of different tumor stages using fundamentally different bioinks
Abstract
Although 2D cancer models have been the standard for drug development, they don’t resemble in vivo properties adequately. 3D models can potentially overcome this. Bioprinting is a promising technique for more refined models to investigate central processes in tumor development such as proliferation, dormancy or metastasis.
We aimed to analyze bioinks, which could mimic these different tumor stages in a cast vascularized arteriovenous loop melanoma model in vivo. It has the advantage to be a closed system with a defined microenvironment, supplied only with one vessel—ideal for metastasis research.
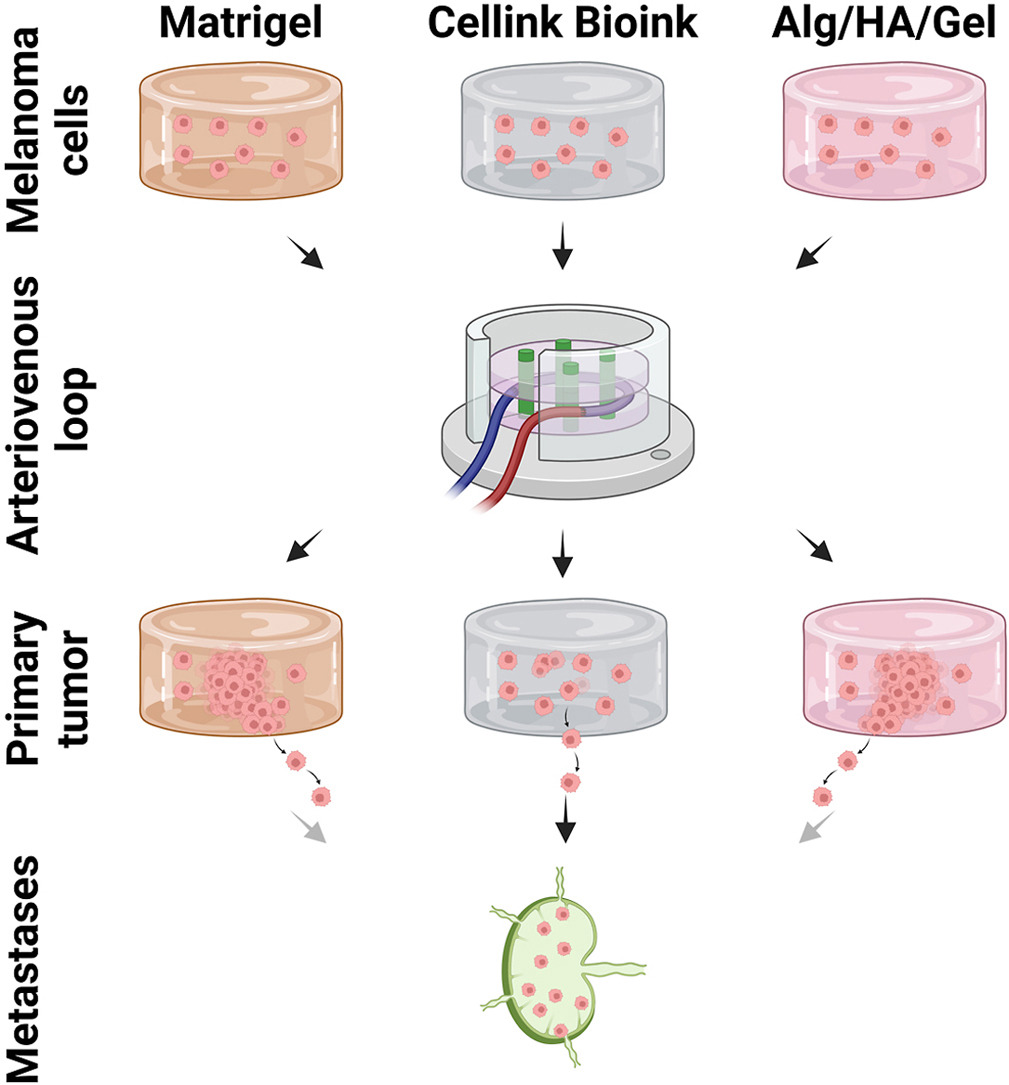
Tested bioinks showed significant differences in composition, printability, stiffness and microscopic pore structure, which led to different tumor stages (Matrigel and Alg/HA/Gel for progression, Cellink Bioink for dormancy) and resulted in different primary tumor growth (Matrigel significantly higher than Cellink Bioink). Light-sheet fluorescence microscopy revealed differences in vascularization and hemorrhages with no additional vessels found in Cellink Bioink. Histologically, typical human melanoma with different stages was demonstrated. HMB-45-positive tumors in progression inks were infiltrated by macrophages (CD163), highly proliferative (Ki67) and metastatic (MITF/BRN2, ATX, MMP3). Stainings of lymph nodes revealed metastases even without significant primary tumor growth in Cellink Bioink.
This model can be used to study tumor pathology and metastasis of different tumor stages and therapies.
Introduction
Melanoma is a highly malignant skin cancer that has its origin in melanocytes. Even small primary tumors typically can metastasize at an early time point. These metastases can be found in skin but also in lymph nodes, lungs, liver and brain [1]. Advanced stages, where a surgical resection is no longer indicated, are targets for novel drugs. With these, prognosis of patients has improved over the last decade. Nevertheless, drug resistances are still a prominent problem. Hence, there is a huge demand for highly complex models to study molecular processes and evaluate and improve therapies.
Tissues and organs are complex, hierarchical structures containing multiple cell types depending on vascularization with increasing size. Similarly, tumors and their microenvironment also consist of various cells and rely on vascularization and angiogenesis to prevent necrosis of larger tumor masses. This tumor microenvironment has been shown to play a crucial role in tumor promotion and in drug receptiveness [2,3]. Hence, complex 3D models have been created to mimic the microenvironment as accurately as possible. These often make use of hydrogels and, in recent years, also printable hydrogels for biofabrication, and offer interesting insight into phenotypical changes caused by the microenvironment [4]. Printable hydrogels with cells, the so-called bioinks, are used for creating the desired structures in a precise and controllable manner. The hydrogel should ideally resemble the extracellular matrix in structure, composition, and biomechanical properties. Therefore, they have to be adapted to the desired application. One standard material often used for cancer models is Matrigel, a basement membrane-like mixture of type IV collagen, laminin, entactin, heparan sulfate proteoglycans, and various growth factors. It allows ideal tumor interaction including adhesion and remodeling. Other commercial products, like Cellink Bioink, are more defined and consist of selected components like in this case nanocellulose and alginate for crosslinking. This combination does not allow active receptor interaction with mammalian cells. Hence other bioinks, that are more favorable for mammalian cells in terms of anchorage, interaction and remodeling, incorporate besides alginate further ECM-derived molecules like gelatin [5] or hyaluronic acid [6]. Recently, a combination of all of these components as a bioink has been proven to be suitable as complex in vivo model [7].
This in vivo model combats one of the major challenges in tissue engineering, the lack of adequate vascularization in larger constructs. The arteriovenous (AV) loop model has been proven to be a very effective option to vascularize a 3D scaffold in a controlled manner in vivo [8]. In this rat model, the femoral artery and vein are anastomosed into an AV loop by interposing a venous graft from the contralateral side of the animal. The resulting vascular loop is put into a chamber that is filled with a hydrogel or bioink. In suitable hydrogels, this vascular loop starts neovascularization by vascular sprouting into the hydrogel over the course of a few weeks, ensuring supply of incorporated cells with nutrients. This makes the AV loop an interesting option also for tissue engineering as the patient’s body could be used as a bioreactor to vascularize a tissue-engineered construct. Bioprinting increases the controllability of the AV loop model e. g. through precise positioning of different cells of the tumor microenvironment or macroporous structures.
With this study, we evaluated the influence of the three different bioinks Matrigel, Cellink Bioink, and Alg/HA/Gel on the growth of primary melanomas and metastases in the AV loop model to further establish it as a robust tumor model.
Download the full article as PDF here A vascularized in vivo melanoma model suitable for metastasis research of different tumor stages using fundamentally different bioinks
or read it here
2.2. Ink composition
Matrigel Basement Membrane Matrix (Corning, Inc., Corning, NY, USA) was used undiluted according to the manufacturer’s protocol. Cellink Bioink (Cellink, BICO Group, Gothenburg, Sweden) was diluted 10+1 with cell culture medium according as indicated by the manufacturer. Alg/HA/Gel was created as previously published [7]. 0.5% m v-1 VIVAPHARM Alginate PH 176 (JRS PHARMA GmbH & Co. KG), 3% m v-1 gelatin from porcine skin (gel strength ≈300 g Bloom, Type A, Sigma-Aldrich), and 0.1% m v-1 hyaluronic acid (1–2 MDa, CarboSynth Ltd, Compton, UK) were dissolved in PBS without Ca2+ and Mg2+ (Sigma-Aldrich) at 37 °C.
Rafael Schmid, Sonja K. Schmidt, Stefan Schrüfer, Dirk W. Schubert, Stefanie Heltmann-Meyer, Martin Schicht, Friedrich Paulsen, Raymund E. Horch, Anja K. Bosserhoff, Annika Kengelbach-Weigand, Andreas Arkudas,
A vascularized in vivo melanoma model suitable for metastasis research of different tumor stages using fundamentally different bioinks, Materials Today Bio, Volume 26, 2024, 101071, ISSN 2590-0064, https://doi.org/10.1016/j.mtbio.2024.101071.
Read also our introduction article on Alginates here:


